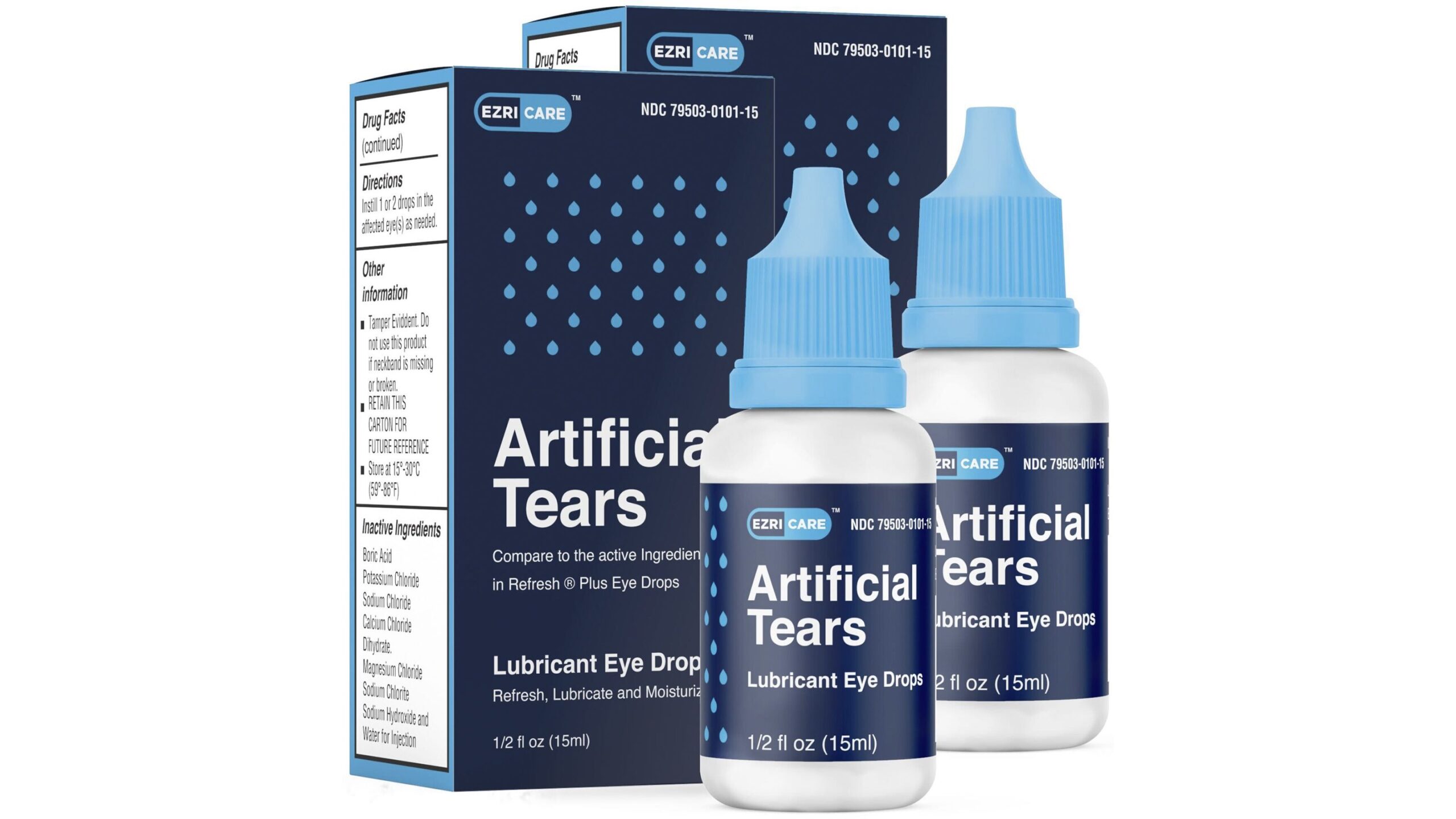
The maker of eyedrops sold under the brand name EzriCare recalled them Thursday because of possible contamination with Pseudomonas aeruginosa, a type of bacterium resistant to most antibiotics.
The EzriCare artificial tears have been linked to at least 55 cases of bacterial infection in 12 states. Five of those people so far have had vision loss. One person died when the bacteria entered the bloodstream.
Global Pharma Healthcare, which is based in India, announced the voluntary recall of its Artificial Tears Lubricant Eye Drops on Thursday. The recall comes after the Centers for Disease Control and Prevention urged people to immediately stop using the eyedrops.
“The product was distributed nationwide in the USA over the internet,” Global Pharma Healthcare wrote in its announcement of the recall.

The drops are distributed by EzriCare and Delsam Pharma. EzriCare said in a statement Wednesday that it “had no role in the formulation, packaging delivery system or actual manufacturing of this product.” The company said it only designed the label and marketed the product.
On Wednesday, the CDC said it is working with the Food and Drug Administration and state and local health officials to investigate the outbreak. A majority of the patients reported using the eyedrops before they became ill.
By
NBC News